EDIT: I hate to inform you all that while the chemistry is correct – the video shown here is entirely faked. Apparently the explosions weren’t exciting enough… http://wp.me/p17eqS-6e
Well, I promised explosions and I cannot disappoint, so welcome to the first installment of Friday Explosions! I’ll start off with a classic, Alkali metals in water. But not those “rubbish” ones like sodium and lithium, these guys blow holes in bathtubs with rubidium and cesium (aka, “The dog’s nuts of the periodic table”). Watch and enjoy. Explanation below.
The How
So! Explosions are cool. But what is exploding? The metal, or the water? The answer is neither of course, because of the following reaction:
Here “M” stands for any alkali metal. As soon as it touches water the alkali metal rips hydrogen off of the water molecule and produces hydrogen gas (H2). There is so much heat released in this reaction that the hydrogen produced actually self-ignites. Yes it self-ignites, while still dissolved in the water. This is the boom process which blows up the bath tubs and sends the rest of the water sky high:
The Why
The astute reader will now wonder: “Aha! If this reaction happens with any alkali metal, why is lithium such rubbish and cesium so explosive?” To answer this, we go back and look at the periodic table.
You’ll notice that all the Alkali metals are grouped up on the left hand side in a column. This means that they all have similar chemical properties. Importantly for our discussion, it means that each of the alkali metals has only one electron sitting waaaaaay far out from the nucleus as compared to the other atoms. How far out? This next figure shows how large all the atoms of each element are relative to one another:
Look how much massively larger (relative to the other elements that is) the highlighted alkali metals are than the other elements. Also note how when going down the periodic table from lithium down to cesium, the atoms get larger and larger. That means that the lone electron is getting further and further away from the center of the atom, and that it is getting easier and easier to rip it off and do interesting (explosive) things with it! This is why cesium is so much more reactive than lithum in water. The negatively charged electron is so far away from the positively charged nucleus that in the presence of water it transfers to the water and makes hydrogen gas extremely rapidly. Conversely the electron on lithium is much closer to the nucleus, is much more tightly held, and it transfers to water very slowly, meaning the hydrogen producing reaction takes much longer to occur. Hydrogen gas never builds up in the water to any appreciable amount, resulting in a disappointing display.
So in a nutshell – the bigger the atom, the electrons come off easier and faster. In this case that results in bigger booms! Have a good weekend and come back next Friday for Spooky Explosions!
– PV

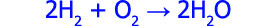


Way cool PV. This will be fun. Can’t wait for next week!
Well done Mr Science!
This is totally rad, PV. I look forward to the future “Explosion Fridays”!
Freakin awesome. We did the friendlier ones in lab when I was in undergrad. They chucked Na and Li into the fountain outside the science building… My favorite is Cs! 🙂
Great job Paul!
Was this on Top Gear?
It’s possible. I think this is a British show called Brainiac.
Where does the oxygen come from? Just the oxygen dissolved in the water?
That was my interpretation. I bet you’d get less bang if you had a way to decrease dissolved oxygen in the water.
I think that the partial pressure oxygen in the water equilibrates with the partial pressure of oxygen in the air pretty quickly. I’ll bet you could get more bang if you bubbled oxygen through the tub of water!
[…] is so explosive because it’s different from the other two substances we looked at previously (alkali metals and acetylene gas). Most people are aware that flames need oxygen to continue to burn. The classic […]